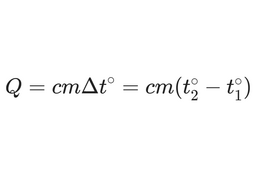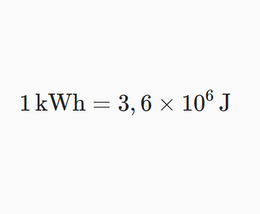- The law of conservation of energy applies to heat transfer.
- The law of conservation of heat holds that heat transfer is governed by the law of conservation of heat.It takes a different amount of heat energy to heat a body of the same mass but made of different materials. The heat energy released when these bodies cool down is also different.
- The heat capacity shows how much heat energy can be stored in a body.
To start this topic, we can talk, for example, about heating costs, which is a topical issue at the moment. Although students do not yet have to pay their own heating costs, it may affect them in other ways, for example through the amount of pocket money.
Students are expected to not be able to answer the question in the first worksheet, but we will come back to it during the lesson.
Next, we begin to calculate the amount of energy transferred from one body to another in heat transfer.
The fact that the law of conservation of energy applies in heat transfer is very important. Of course, it is also a very fundamental and therefore quite abstract law, but we take it into account in all our calculations, which is why it is reasonable to discuss it once in its pure form.
We learn to calculate how much energy a lamb has in its bodies.
It is important to emphasize that we do not calculate how much heat is in a body in total. We calculate how much energy needs to be added to the body or how much energy is released when the body's temperature changes by a certain number of degrees.
Let's start by trying to establish hypotheses about which properties of the body depend on the amount of heat stored in them.
Moving step by step, you could next familiarize yourself with the concept of specific heat of a substance and try to connect it with the discussions on the previous slide.
Now the formula or model:
In the following worksheet, we will also try to use the formula for calculating the amount of heat. Hopefully, based on the above, the students will understand what is meant by this formula and why the formula looks like this.
The worksheet can also calculate the amount of heat by entering the corresponding parameters, thanks to which it is possible to quickly calculate the amount of heat released or absorbed during the cooling or heating of different bodies or to check the result of your own calculations.
In the following, we will try to answer the question about the cost of taking a shower in the interest generator. For this we need to start with units of energy.
Nobody likes converting units, but there's no other way to deal with real life than to do it anyway. It is a fact that electricity bills say kilowatt-hours, not joules.
Students probably already know how to use various unit conversion calculators that can be found through google. If you write " J to kwh " in the search, the first match is a perfectly good and reasonable calculator. We think that the ability to use such aids is something that could be used when dealing with more complex units, such as kilowatt-hours.
For the sake of completeness of our study materials, we have also created one such simple calculator. In any case, the topic given here should be discussed with the students.
Now, finally, the price calculation:
At this point, it is also important to create a connection between the study of heat and what was learned in mechanics, that is, to remind us what we already know about the physical quantity called energy. Therefore, you have to remember what you learned in the mechanics course.
Content integration is undoubtedly important, and here is where it is especially necessary to address it. At the same time, making such connections is difficult for many students, so in each specific situation it should be decided separately whether the use of the following worksheets is appropriate or not.