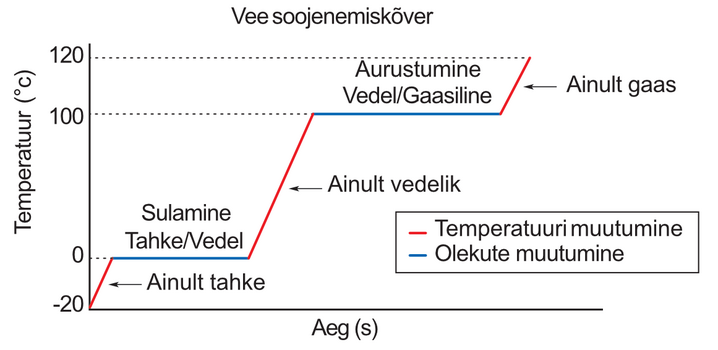
Use the video to watch an experiment in which ice turns into water vapor when continuously heated. Make sure the ice/water temperature is constant during melting and evaporation.
We see that when ice melts and water evaporates, the temperature remains at the melting point ( degrees) and the boiling point ( degrees), respectively. At the same time, the stove continues to work, so the heat transfer is not interrupted. Where does this heat go? This is a legitimate question because we already know that if a substance absorbs heat, its temperature should rise.
In answer to this question, it must be said that heat is used to change the state of the substance, i.e. to push the molecules of the substance further apart (recall what we saw in the simulations).
How much energy is needed to melt one kilogram of a substance is a constant specific to each substance (heat of fusion). You can look it up in the table.
A careful observer can see that the temperature still seems to rise slightly as the ice melts in the video. This is because the center of the ice, where the temperature is measured, warms up to degrees over a longer period of time than the edges of the piece of ice. The jumps in the graph are caused by the fact that the ice begins to fall off the tip of the temperature sensor in the final stages of melting.