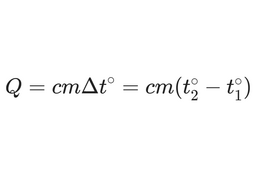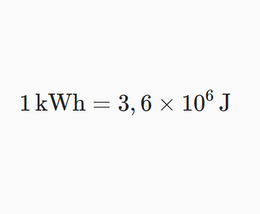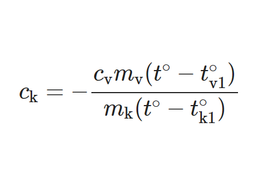- The law of conservation of energy applies to heat transfer.
- The law of conservation of heat holds that heat transfer is governed by the law of conservation of heat.It takes a different amount of heat energy to heat a body of the same mass but made of different materials. The heat energy released when these bodies cool down is also different.
- The heat capacity shows how much heat energy can be stored in a body.
To start this topic, we can talk, for example, about heating costs, which is a topical issue at the moment. Although students do not yet have to pay their own heating costs, it may affect them in other ways, for example through the amount of pocket money.
Students are expected to not be able to answer the question in the first worksheet, but we will come back to it during the lesson.
Next, we begin to calculate the amount of energy transferred from one body to another in heat transfer.
The fact that the law of conservation of energy applies in heat transfer is very important. Of course, it is also a very fundamental and therefore quite abstract law, but we take it into account in all our calculations, which is why it is reasonable to discuss it once in its pure form.
We learn to calculate how much energy a lamb has in its bodies.
It is important to emphasize that we do not calculate how much heat is in a body in total. We calculate how much energy needs to be added to the body or how much energy is released when the body's temperature changes by a certain number of degrees.
Let's start by trying to establish hypotheses about which properties of the body depend on the amount of heat stored in them.
Moving step by step, you could next familiarize yourself with the concept of specific heat of a substance and try to connect it with the discussions on the previous slide.
Now the formula or model:
In the following worksheet, we will also try to use the formula for calculating the amount of heat. Hopefully, based on the above, the students will understand what is meant by this formula and why the formula looks like this.
The worksheet can also calculate the amount of heat by entering the corresponding parameters, thanks to which it is possible to quickly calculate the amount of heat released or absorbed during the cooling or heating of different bodies or to check the result of your own calculations.
In the following, we will try to answer the question about the cost of taking a shower in the interest generator. For this we need to start with units of energy.
Nobody likes converting units, but there's no other way to deal with real life than to do it anyway. It is a fact that electricity bills say kilowatt-hours, not joules.
Students probably already know how to use various unit conversion calculators that can be found through google. If you write " J to kwh " in the search, the first match is a perfectly good and reasonable calculator. We think that the ability to use such aids is something that could be used when dealing with more complex units, such as kilowatt-hours.
For the sake of completeness of our study materials, we have also created one such simple calculator. In any case, the topic given here should be discussed with the students.
Now, finally, the price calculation:
At this point, it is also important to create a connection between the study of heat and what was learned in mechanics, that is, to remind us what we already know about the physical quantity called energy. Therefore, you have to remember what you learned in the mechanics course.
Content integration is undoubtedly important, and here is where it is especially necessary to address it. At the same time, making such connections is difficult for many students, so in each specific situation it should be decided separately whether the use of the following worksheets is appropriate or not.
Soojushulk. Erisoojus: tähtsad laused
Teadmiste kontroll: soojushulk, erisoojus
- Mis on soojushulga määramise katsed ehk nn kalorimeetrilised katsed ning kuidas neid läbi viiakse.
- Millised on kalorimeetriliste katsete rakendusvaldkonnad.
In the following introductory slide, experiments are proposed to determine both the specific heat and the nutritional value of food. It is clear that both experiments cannot be done in one hour, and a decision must be made.
The nutritional value test also produces a considerable amount of fat, which is why it might make more sense to study it using a video instead. In any case, one could hope that the answer to the question "Does one or another food have a good effect on my weight?" could encourage students to think along.
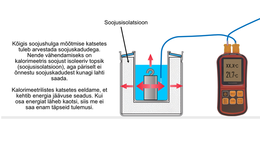
Ideally, when performing calorimetric experiments, students could also understand why these experiments are performed in this way. Thanks to this, they would also be able to manage their own activities, and not just act based on the points of the work manual. However, this is not easy to achieve, because practically all the knowledge learned in the previous lessons comes into play. That's why we start step by step, not just by handing over work instructions.
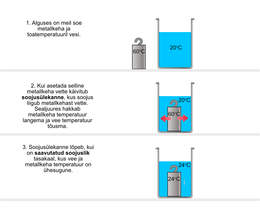
Let us first recall how well-known phenomena are explained through the terms we discussed earlier. So: how do you explain the cooling of a metal body placed in water through "heat transfer" and "thermal balance". Then it is easier to start explaining the general principle of calorimetric experiments.
Next, we take up the generalized form of the principle of calorimetric experiments with a definition. The worksheet also asks for the possibility to calculate the amount of heat given off by the test object. For this purpose, the test object must be weighed, if this has not been done before. Designing such "holes" in students' paths is beneficial because it fosters the ability to think and make decisions independently.
The next worksheet also provides a mathematical derivation of the final formula describing the experiment. This could be taken as an example of how physicists themselves use physical models, mathematical transformations and final formulas of various forms. It could be affordable for better students.
Let's start the preparation of the real experiment by talking about heat losses in calorimetric experiments. However, this topic can also be moved to the discussion of test results, or even skipped altogether.
We offer three options for conducting the test.
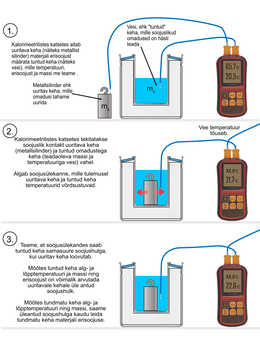
The first option tries to carry out the work in a way that once again emphasizes the idea of the method - the amount of heat given off by the body under study increases the temperature of the known body, which in turn allows us to calculate the specific heat of the material of the body under study. In this case, the experiment and the analysis of its results are divided into three worksheets.
First, a worksheet for conducting the experiment.
Next, we calculate the amount of heat transferred to the water in the calorimeter.
Finally, using the amount of heat calculated in the previous worksheet and the test data, we calculate the specific heat of the body material (aluminum) under study.
The worksheet of the second variant uses the final formula derived above to calculate the specific heat of the material of the test object.
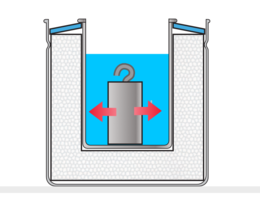
The worksheet of the third variant is structured in a way that allows measuring the specific heat of a test object made of any material. In doing so, the effect of the inner aluminum cup of the calorimeter on the test result is also taken into account.
Determination of the specific heat of the test object material considering the heat capacity of the calorimeter cup
In this subsection, there are materials that allow us to relate the heat teaching material to topics that are close to the students: the calories hidden in foods, that is, the nutritional value of foods. These topics are also dealt with in other courses, which is why the integration with physics is absolutely appropriate.
On the first slide, we remind you of the relationship between a calorie and a joule.
Try it yourself: put a nut or cereal on the fire and see how much the water heats up. So then we try to calorimetrically measure the chemical energy released during combustion.
The test is "dirtier" than the cooling/heating of the test object - smoke is emitted and the worktops have to be cleaned later.
Kalorimeetrilised katsed: tähtsad laused
Teadmiste kontroll: kalorimeetrilised katsed I
Teadmiste kontroll: kalorimeetrilised katsed
Siit see siis lõpuks tuleb.
Igaks-juhuks tuletame esmalt meelde eespool juba kasutatud töölehte, mis tutvustab energiaühikut kilovatt-tund, selle teisendamist džaulideks ning küsib mõned küsimused.
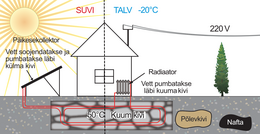
Edasi siis meie suur küsimus: „Kas Eestis on võimalik maa sisse salvestatud soojusega talvel maja ära kütta?"
Alustuseks peaksime hindama kui palju energiat talviseks kütmiseks kulub.
Selge see, et õpilased oma kodu energiatarbimist ei tea ... arvatavasti teavad seda harva isegi küttearveid maksvad isikud ise. Siiski saame oma arutluste ja hinnangute aluseks võtta teatud standardid, mida nimetatakse energiaklassideks. Järgnev tööleht on mõeldud just selle jaoks.
Kui energiavajadus on teada, saame varasemaid teadmisi kasutades arvutada kas ja kuhu seda salvestada saaks.
"Liivapatareid" saaks kasutada taastuvenergia salvestamiseks?
Selle teema lõpetuseks oleks ehk paslik meelde tuletada kursuse algusest pärit töölehte, milles siin käsitletud küsimuste kohta arvamust avaldasime.
Energia ja võimsus on teemad, mis on üliolulised nii Eesti kui ka terve inimkonna tulevikku määravate küsimuste aruteludes. Näiteks on rohepöörde läbiviimiseks vaja taastuvenergiaallikaid kasutades toota mingi kindel kogus energiat, mistõttu oleks meil vaja teada, kas ja kuidas see taastuvenergiallikaid kasutades võimalik on. Lisaks räägime energiast ka näiteks toitumisega seotud küsimustes.
Siiski on põhikooliõpilastel päris keeruline aru saada näiteks keskmise ja hetkvõimsuse vahelisest erinevusest. Lisaks on raske mõista, et energia jäävuse seadus kehtib tõepoolest kogu looduses ühtviisi - füüsikast bioloogiani, matemaatiliset pendlist inimesteni.
Proovime siin pakkuda töölehti, mis võimaldavad tegeleda oluliste teemadega piisavalt lihtsal ning õpilasete jaoks olulisel viisil.
Alustame sissejuhatavate töölehtedega - neid on siin välja pakkuda kaks.
Esimene neist viitab küsimusele: „Mida päikesepatareidega saab ja ei saa teha?"
Teine variant mängib kulinaaria valdkonna suurte numbritega.
Alustame sellest, et defineerime soojusõpetusele kohasel viisil võimsuse ja teeme mõned arvutused, mis peaksid näitama võimsuse mõiste ... võimsust.
Küttekeha võimsus näitab kui palju soojusenergiat küttekeha ajaühikus soojusvahetuses kaotab/edasi annab. Tavaelus tajume me seda võimsust halvasti - radiaator lihtsalt seisab nurgas ning justkui ei tee mitte midagi ... ja siis tuleb küttearve, ei-tea-millest. Proovime siinkohal tekitada arusaama sellest, et soojus tõepoolest liigub. Seda liikumist on võimalik üheselt ja täpselt mõõta ning võimsus vattides on praktiline info, mida maailma eri osade kohta teada.
Esimeses katses uurime vee jahtumist. Mõõdame vee temperatuuri muutumist ajas ning selle järgi saame teada, milline on võimsus, millega selline veega topsik tuba kütab.
Teeme nüüd ka katse.
Katse läbiviimiseks pakume välja kaks metoodikat.
Esimeseks metoodikaks on nn ühiseksperiment. Nimelt selgitatakse katse alguses klassile, et me teeme seda katset koos: õpetaja ütleb, millal katse algab ning millal tuleb temperatuuri esimest korda mõõta. Kui aeg on läbi, kordame ning mõõdame temperatuuri ka teist korda. Nüüd saame vee massi mõõta (lahutades veega anuma kogumassist anuma massi) ning viia läbi rehkendused.
Kasulik on katse koos õpilastega läbi teha ehk panna katse ka õpetaja lauale „käima," võtta lugemeid, rehkendada jne. See annab õpilastele võimaluse aeg-ajalt piiluda, mida õpetaja teeb.
Tulemusi peaks tahvlil võrdlema ning koos analüüsima. Informatiivne on koos vaadata õpilaste poolt arvutatud võimsust ning topsis oleva vee algtemperatuuri. Üldiselt tuleb välja seaduspärasus, mille kohaselt kõrgem vee temperatuur tähendab kiiremat jahtumist. Seda saab õpilastele selgitada: soojemaks köetud maja kaotab soojust alati kiiremini, st talvel tähendab kõrgem toatemperatuur suuremaid küttearveid.
Teises metoodikas koguvad kõik õpilased piisavalt andmeid selleks, et jahtumise kiiruse ning seega ka kütmise võimsuse kohta järeldusi teha.
Mõned ilmekad arvutused.
Ka võimsuse mõistega oleme mehaanikas juba kokku puutunud. Proovime siin paralleele tuua, teades, et igasugune mehaaniline energia muutub lõppude lõpuks soojuseks.
Niisiis on suvise soojuse salvestamine võimalik, ent see energia tuleb kusagilt saada. Selge see, et tahame selle energia saada taastuvenergiaallikatest.
Proovime järgnevas teha mõned rehkendused, et mõista, kuidas see tegelikkuses töötaks.
Kõigepealt tutvume päikesekiirguse näitel ideega, mille kohaselt saab energiat arvutada teades, kui suur on energiavoo võimsus ruutmeetri kohta.
Edasi nendime, et sellised andmed - maksimaalne võimalik aastane keskmine võimsus ühe või teise taastuvenergiaallika jaoks - on olemas kõigi taastuvenergiaallikate jaoks. Järelikult saab misiganes taastuvenergiaallikaga toodetavat energiahulka arvutada, kui teame, kui suure võimsusega see taastuvenergiaallikas ühe ruutmeetri kohta energiat toodab.
Lõpuks sõlmime otsad kokku ja arvutame, kui suur peab minimaalselt olema meie päikese- või tuulepark, et püstitatud eesmärk - koguda meie majapidamises kogu talveks vajaminev energia suvel kokku - oleks realiseeritav.
Veel üks mõttemäng.
Kassipildi energeetika
Power
Võimsus on füüsikaline suurus, mis on arvuliselt võrdne ajaühikus kulutatud energiaga. Võimsust tähistatakse tähega ning seda saab arvutada valemiga:
Võimsuse ühikuks on vatt (lühend ): 1W=1J/1s.